Anodising guide - 2. Basics
|


|
2.1 Properties of the layer
Polished aluminum oxidizes in air very quickly but becomes covered with an air-impermeable
colorless oxide (Al2O3). This very thin (typically 0.5-1 micron thick) layer protects the metal from
further attacks of atmospheric oxygen. It is quite soft and unsightly and isn't acceptable for more advanced
requirements because of its very low strength. However, using an aqueous electrolyte you can build up this
layer much more giving it the following features:
- Hardness: Mohs hardness of about 9 (corundum), quartz has hardness 7, diamond has hardness 10
- Thickness: depending on voltage, anodising duration and other parameters - 5-250 microns (0.005-0.25 mm)
- Dielectric strength: about 30 volts per micron
- Color: colorless to grayish/blackish (depending on the alloy), with suitable metallic alloys the metallic
look of the underlying aluminum is preserved - even at a later coloring.
- Light-resistant and insensitive to scratches. Possible combinations of dyes allow to create almost any
desired color.
2.2 Chemical reaction
The following main chemical reactions are running in the anodising bath:
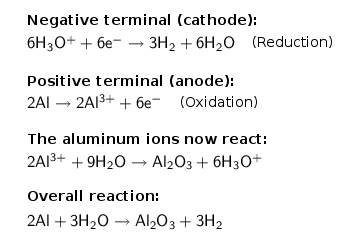
There are two things apparent:
- The formation of the layer itself do not consume sulfuric acid. It serves as the electrolyte (this
massively reduces the electrical resistance of the bath) and - this is the important part - to solve the
layer locally, thus ensuring a continuous current flow and building of layer structure. Due to the small layer
thickness a refreshing of sulfuric acid (recovery of initial concentration of 15-20%) is rarely necessary.
- At the cathode (negative terminal) gaseous hydrogen is generated, which in large quantities should be removed
because of the explosion risk (hydrogen is odorless, tasteless and colorless!).
2.3 Structure of the layer
First in sulfuric acid a closed layer is electrolytically produced which grows further to fine capillary pores:
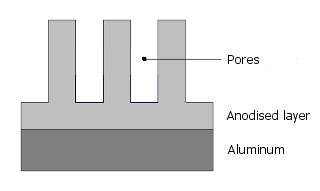
The dye molecules now migrate into these pores:
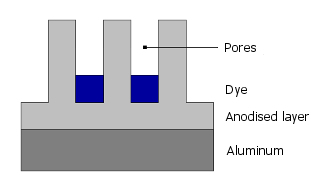
Finally, the pores are closed by sealing.
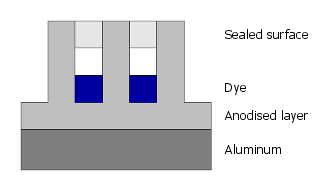
So the dye is not applied on but is part of the anodized layer itself and thus protected very well from abrasion
and chemical attack.



